References
1BRIUMVI® Summary of Product Characteristics.
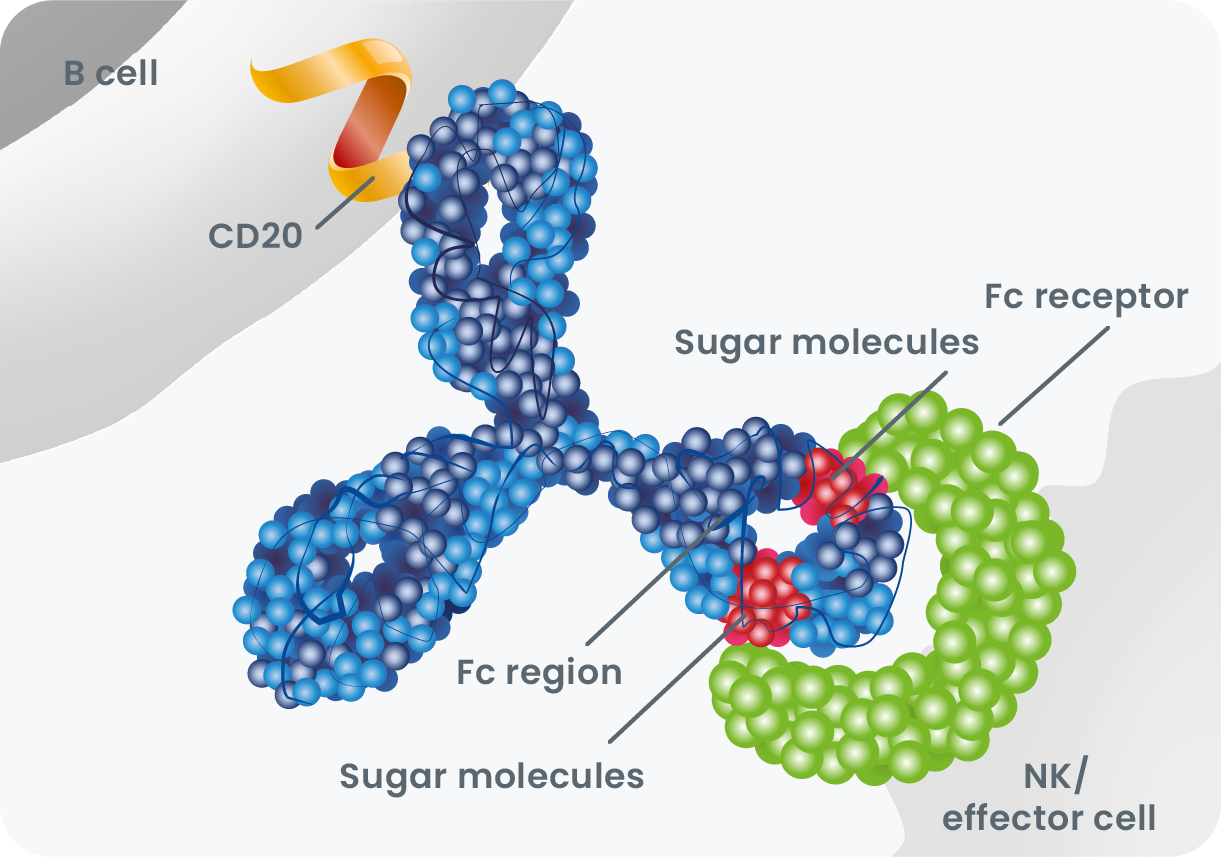
2Ferrara C, Grau S, Jäger C, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core 1 fucose Proc Natl Acad Sci U S A. 2011;108(31): 12669-12674. doi: 10.1073/pnas.1108455108.
3Sun Y, Izadi S, Callahan M, et al. Antibody-receptor interactions mediate antibody-dependent cellular cytotoxicity. J Biol Chem. 2021; 297(1): 100826. doi:10.1016/j.jbc.2021.100826.
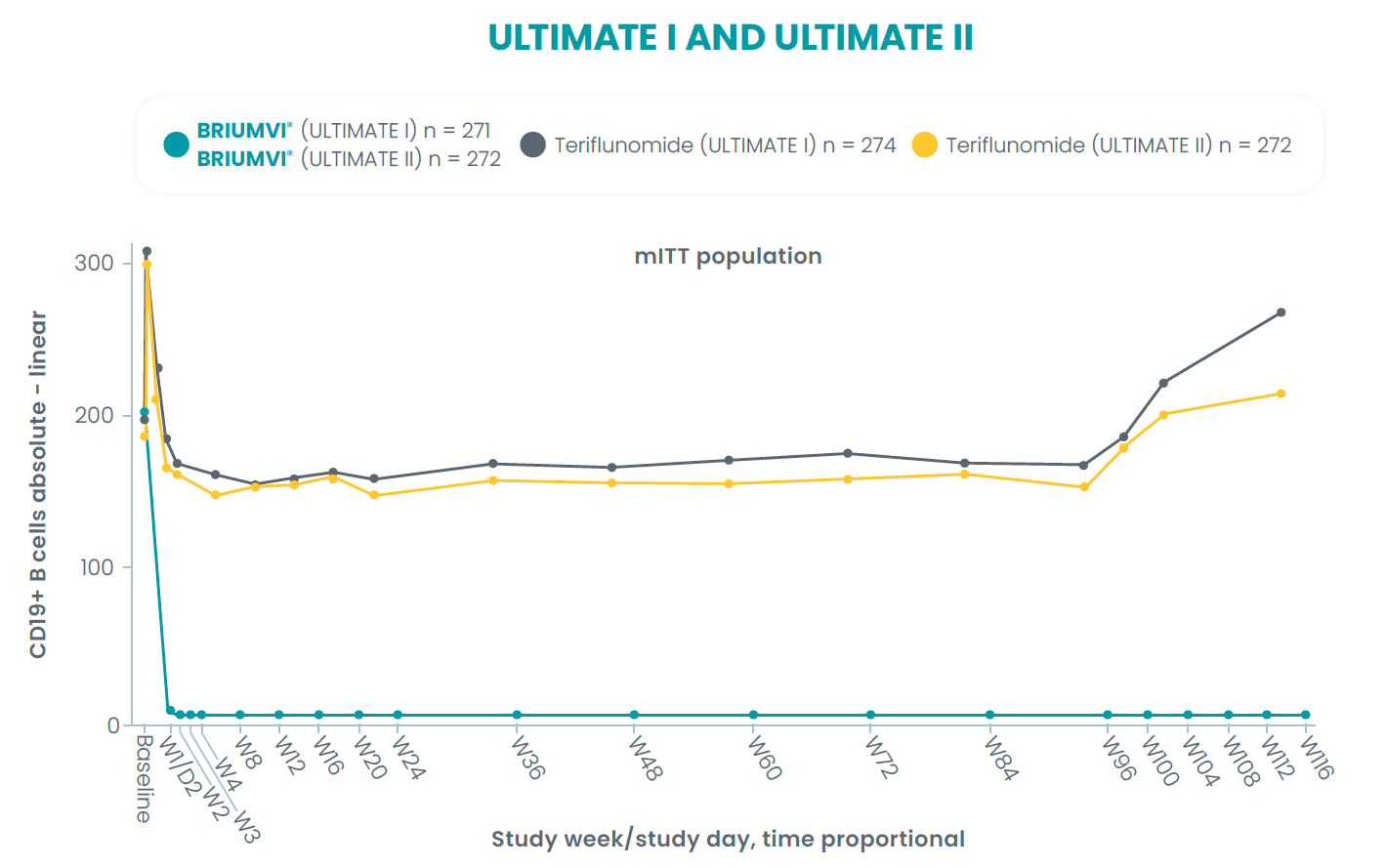
4Steinman L, Fox E, Hartung H-P, et al. Ublituximab versus teriflunomide in relapsing multiple sclerosis. N Engl J Med. 2022; 387(8): 704-714. doi:10.1056/NEJMoa2201904.
5Fox E, Lovett-Racke AE, Gormley M, et al. A phase 2 multicenter study of ublituximab, a novel glycoengineered anti-CD20 monoclonal antibody, in patients with relapsing forms of multiple sclerosis. Mult Scler. 2021;27(3):420-429. doi:10.1177/1352458520918375.
6de Romeuf C, Dutertre C-A, Le Garff-Tavernier M, et al. Chronic lymphocytic leukaemia cells are efficiently killed by an anti-CD20 monoclonal antibody selected for improved engagement of FcRIIIA/CD16. Br J Haematol. 2008; 140(6): 635-643. doi:10.1111/.1365-2141.2007.06974.x.